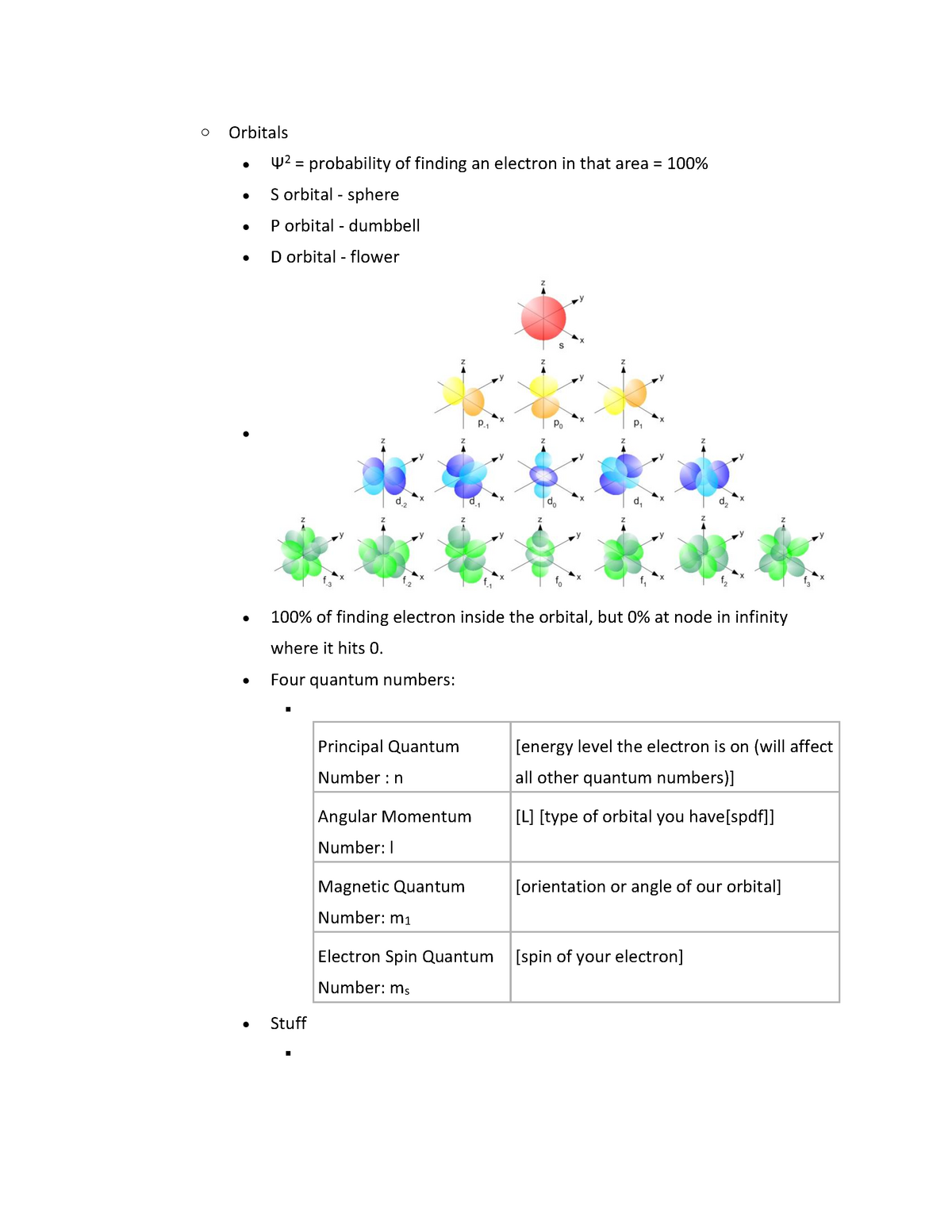
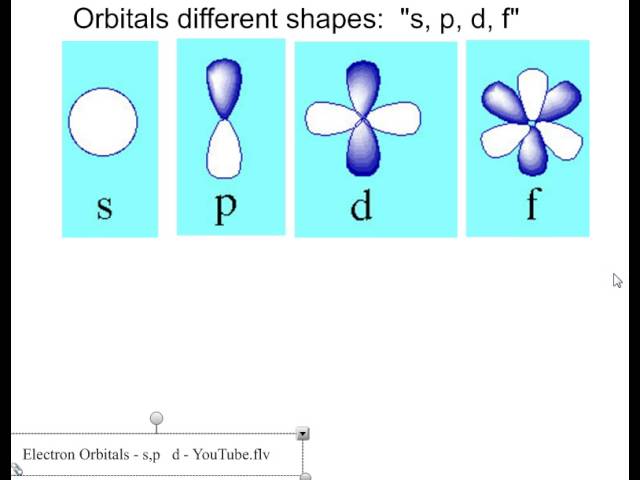
√完了しました! s p d f g h i orbitals 221438-S p d f orbitals
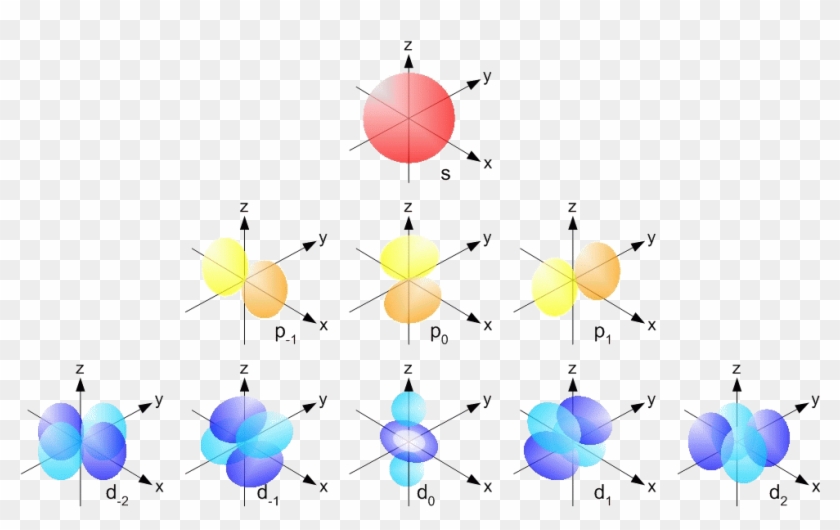
The s orbital, p orbital, d orbital, and f orbital refer to orbitals that have an angular momentum quantum number ℓ = 0, 1, 2, and 3, respectively The letters s, p, d, and f come from the descriptions of alkali metal spectroscopy lines as appearing sharp, principal, diffuse, or fundamentalS, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;Subshells are usually identified by their n and values n is represented by its numerical value, but is represented by a letter as follows 0 is represented by 's', 1 by 'p', 2 by 'd', 3 by 'f', and 4 by 'g' For instance, one may speak of the subshell with n = 2 and as a '2s subshell' The shapes of orbitals Any discussion of the shapes of electron orbitals is necessarily imprecise
Parsing Spdf Orbital Hybridization And Simple Bonding
S p d f orbitals
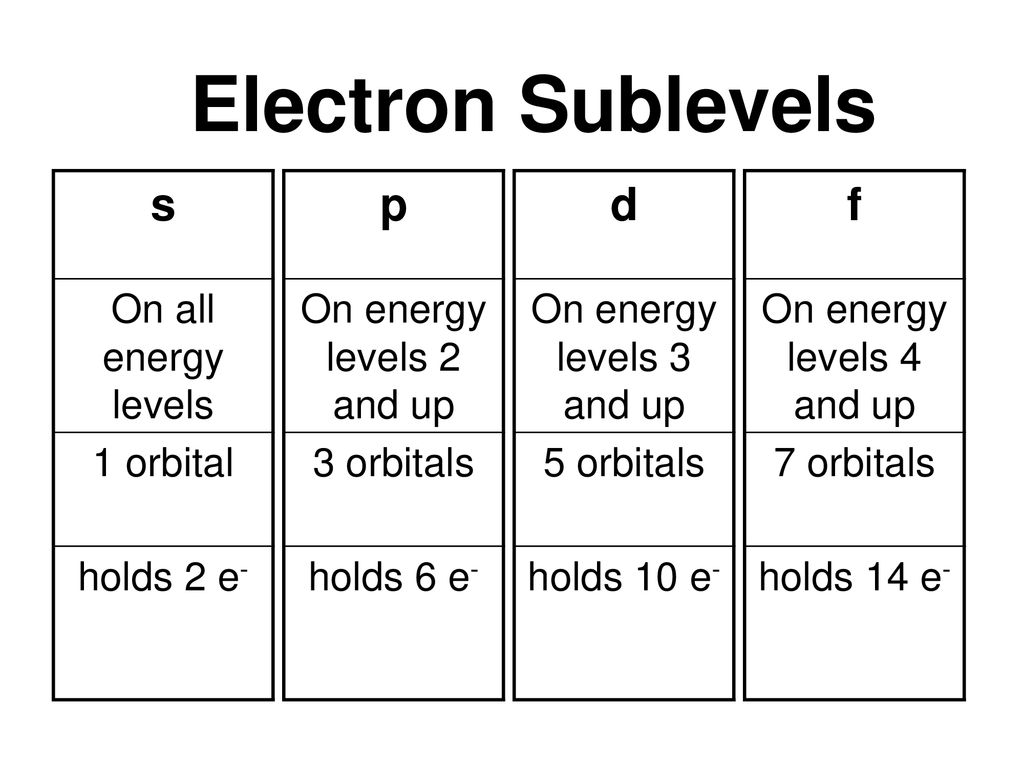
S p d f orbitals-The nomenclature (S, P, D, F) is derived from the characteristics of the spectroscopic lines corresponding to (s, p, d, f) orbitals sharp, principal, diffuse, and fundamental;The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells As shown in Table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes Each of these lobes is labeled differently and is named depending on which plane the lobe
:max_bytes(150000):strip_icc()/ShellAtomicModel-5a6ab592aded4bb7a1328f809e4f10da.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers
Therefore, elements can have up to seven energy levels, depending on how many electrons the element possessOrbitals Chemistry (s, p, d, and f Orbital) Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry Learn more about atomic orbital at ByjusAn electron in a p orbital has equal probability of being in either half The shapes of the other orbitals are more complicated The letters s, p, d, f, originally were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental, before the relation between spectra and atomic electron configuration was
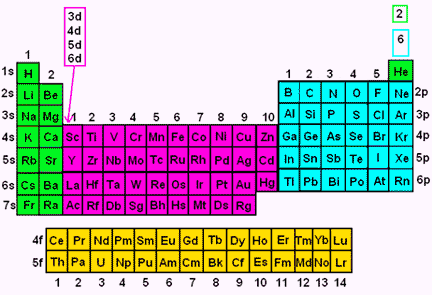
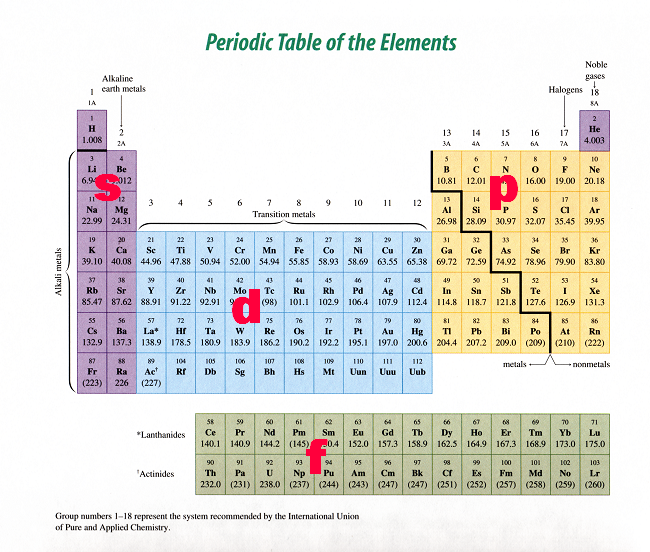
S P D F G H I J s orbitals I Choose 1 forbitals Choose Choose l d orbitals porbitals2s is lower energy than 2p)(image source)So for example,The periodic table consists of four blocks of elements which correspond to the s, p, d, and f orbitals being filled After f orbitals come g and h orbitals In theory, if a g block and h block of elements existed, how long would the rows of g and h elements be in this theoretical periodic table Why?
Question 6 4 pts At n = 3 how many of each type of orbital below exists?The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells As shown in Table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes Each of these lobes is labeled differently and is named depending on which plane the lobeSublevel s contains one orbital, p contains three, d has five, f has seven, g has nine, h has 11 and i has 13 The periodic table of elements contains seven rows;


Orbitals Lecture Notes 6 Warning Tt Undefined Function 32 Warning Tt Undefined Studocu



Why Different Orbitals Have Different Shapes Britannica
Footnotes (1) Each subshell is made up of a set of orbitals, the orbitals reflect which subshell they belong to by using the same letter, that is, there are s orbitals, p orbitals, d orbitals and f orbitals However, although there is only one s orbital in the s subshell, there are 3 p orbitals in the p subshell, 5 d orbitals in the d subshell, and 7 f orbitals in the 5 subshellThe effect of a crystal field on different orbitals in an octahedral field environment will cause the d orbitals to split to give t 2g and e g subsets and the D ground term states into T 2g and E g, (where upper case is used to denote states and lower case orbitals) f orbitals are split to give subsets known as t 1g, t 2g and a 2gAn electron in a p orbital has equal probability of being in either half The shapes of the other orbitals are more complicated The letters s, p, d, f, originally were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental, before the relation between spectra and atomic electron configuration was
/4fz3-electron-orbital-117451436-587f69f23df78c17b6354ebd-f7499851032246f5bbe03f1ffba963d5.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers


How Do You Draw S P D F Orbitals Socratic
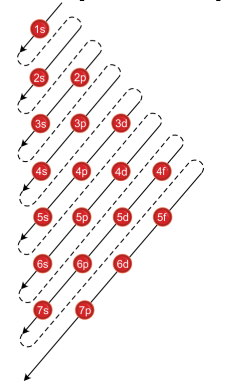
There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental) Within each shell of an atom there are some combinations of orbitals In the n=1 shell you only find s orbitals, in the n=2 shell, you have s and p orbitals, in the n=3 shell, you have s, p and d orbitals and in the n=4 up shells you find all four types of orbitalsOther articles where Gorbital is discussed crystal Ionic bonds s, p, d, f, g, and so forth Each subshell is divided further into orbitals) Two electrons are transferred from the cations to the anions, leaving each with a closed shell The alkaline earth chalcogenides form ionic binary crystals such as barium oxide (BaO), calcium sulfide (CaS), barium selenideThe chemical properties of an element are mostly due to the number of electrons in the s and p subshells, and the different types hold different numbers of electrons 2 in s, 6 in p, 10 in d, 14 in f, and so on (increasing by 4 each time) The lines on the periodic table loop around when the s and p subshells are filled


How Do You Draw S P D F Orbitals Socratic


Shape Of Orbitals Spdf Download Online Games
Orbitals Chemistry (s, p, d, and f Orbital) Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry Learn more about atomic orbital at ByjusAfter two rows of s p and dblock elements, the "f" block opens up with cerium $\ce{4f^1 5d^1 6s^2}$ So we'd need two more rows of the fblock (ie, the lanthanides and actinides) and then complete the row Right now, we have found element 118, so if we can synthesize a few more, we can open up the "g" blockThe symbols s, p, d, and f were subsequently taken over and applied to the orbitals themselves once quantum mechanics actually derived the existence of these levels After f, orbitals are simply labelled alphabetically, so the sequence is s, p, d, f



What Is Spdf Configuration Chemistry Stack Exchange



Parsing The Spdf Electron Orbital Model Chemistry Education Chemistry Classroom Teaching Chemistry
S p d f g h The subshell with n=2 and l=1 is the 2p subshell;S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;2s is lower energy than 2p)(image source)So for example,


How To Use Spdf Method For Electronic Configuration Chemistry Topperlearning Com 8ekub122



Electron Shell Wikipedia
Fred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do withYou will see the lowercase letters s, p, d, f, g, and h for the suborbitals For example, the electron in a hydrogen (H) atom would have the values n=1 and l=0 The single electron would be found in the "K" shell and the "s" suborbital If you go on to learn more about chemistry, you may see its description written as 1s1If n=3 and l=0, followed by a superscript to indicate how many electrons are in the set of orbitals (eg, H 1s 1) Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square


Quantum Model And Spdf Orbitals Youtube
:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)


S P D F Orbitals And Angular Momentum Quantum Numbers
3D model to visualise the shapes of atomic orbitals s, p and dTherefore, elements can have up to seven energy levels, depending on how many electrons the element possessIn the periodic table the heaviest element Z1, orbitals g h i will be used the subshells s p d are like empty jars to fill up jelly beans, when one jar is filled, you need another jar to fill up more electrons


Parsing The Spdf Electron Orbital Model


Shape Of Orbitals Spdf Download Online Games
If n=3 and l=0, it is the 3s subshell, and so Thus the s subshell has only one orbital, the p subshell has three orbitals, and so on 4 Spin Quantum Number (m s) m s = ½ or ½ Specifies the orientation of the spin axis of an electron An electron can spin inLetter s p d f g h The subshell with n=2 and l=1 is the 2p subshell;Although there is no pattern in the first four letters (s, p, d, f), the letters progress alphabetically from that point (g, h, and so on)Some of the allowed combinations of the n and l quantum numbers are shown in the figure below The third rule limiting allowed combinations of the n, l, and m quantum numbers has an important consequence It forces the number of subshells in a shell to be



File Atomic Orbitals Spdf M Eigenstates Mpositive Png Wikimedia Commons
:max_bytes(150000):strip_icc()/ShellAtomicModel-5a6ab592aded4bb7a1328f809e4f10da.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers
If n=3 and l=0, followed by a superscript to indicate how many electrons are in the set of orbitals (eg, H 1s 1) Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square2s is lower energy than 2p)(image source)So for example,S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;


Shells Subshells And Orbitals Video Khan Academy



Spdf Orbitals Location Diagram Quizlet
Sublevel s contains one orbital, p contains three, d has five, f has seven, g has nine, h has 11 and i has 13 The periodic table of elements contains seven rows;The empty f orbitals in lanthanum, actinium, and thorium contribute to chemical bonding, as do the empty p orbitals in transition metals 32 Vacant s, d, and f orbitals have been shown explicitly, as is occasionally done, 33 to emphasise the filling order and to clarify that even orbitals unoccupied in the ground state (eg lanthanum 4f or2s is lower energy than 2p)(image source)So for example,



S P D F Orbitals


Electron Shells And Orbitals Stone Cold Chemistry Talk
4s = 2 4p = 6 4d = 10 4f = 14 32 Total ElectronsSublevel s p d f g* h* *These sublevels are not used in the ground state of any known element • Each sublevel is given a letter designation (like s) and a number designation (s = 0)The s orbital, p orbital, d orbital, and f orbital refer to orbitals that have an angular momentum quantum number ℓ = 0, 1, 2, and 3, respectively The letters s, p, d, and f come from the descriptions of alkali metal spectroscopy lines as appearing sharp, principal, diffuse, or fundamental



Parsing The Spdf Electron Orbital Model High School Chemistry Teaching Chemistry Chemistry


Parsing The Spdf Electron Orbital Model
Shapes of Orbitals and Electron Density Patterns The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z) It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily)However, if you look at a crosssection of an orbital, it isn't uniform6s 6p 6d 6f Level 7 7s 7p 7d 7f Higher orbitals gorbitals 5g 6g 7g horbitals 6h 7h iorbitals 7i Hybrid orbitals coming soon Molecular orbitals coming soon The Orbitron A gallery of atomic orbitals and a few molecular orbitals Images representing atomic orbitals and a few molecular orbitals;These letters are s, p, d, f, g, h, I, j, and many more but here, we are only looking at letters p, s, and d and their corresponding shapes You should know that it is impossible to draw an orbital because an electron is capable of taking up all space but we can draw the shape that it takes up most of the time, presumably 90% of the time


Shape Of Orbitals Spdf Download Online Games


4 Quantum Numbers Of An Electron Orbisophchemistry P And D Orbitals Free Transparent Png Clipart Images Download
Letter s p d f g h The subshell with n=2 and l=1 is the 2p subshell;S p d f g h The subshell with n=2 and l=1 is the 2p subshell;Hund's Rule All orbitals of a given sublevel must be occupied by single electrons before pairing begins Equilibrium or Chemical Equilibrium A state of dynamic balance in which the rates of forward and reverse reactions are equal, the state of a system when neither forward or reverse reaction is thermodynamically favored


Q Tbn And9gcth1rc3hbnde1titk095wzz5fdzyo5obndscg8azgis25 Lq4re Usqp Cau


Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
A s orbital _ 2__ b the subshell of p orbitals __ 6___ c the subshell of d orbitals _ 10 __ d the subshell of f orbitals__ 14 __ e the subshell of g orbitals__ 18 __ 10 How many electrons can inhabit all of the n=4 orbitals?S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;Orbitals with l = 0 are called s orbitals and they make up the s subshells The value l = 1 corresponds to the p orbitals For a given n, p orbitals constitute a p subshell (eg, 3p if n = 3) The orbitals with l = 2 are called the d orbitals, followed by the f, g, and horbitals for l = 3, 4, and 5


Electron Configuration Wyzant Resources


Parsing Spdf Orbital Hybridization And Simple Bonding
The orbitals are of 4 types They are named s,p,d,f The s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively The letters and words refer to the visual impression left by the fine structure of the spectral lines whichD and f orbitals In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z) At the third level there are a total ofThe letter refers to the shape of the orbital The letters go in the order s, p, d, f, g, h, i, j, etc The letters s, p, d, and f were assigned for historical reasons that need not concern us All we have to do is remember the shapes that correspond to each letter



Spdf Trick To Remember It Easily Youtube
:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers
The rest being named in alphabetical order from G onwards, except that J is omittedWhen used to describe electron states in an atom, the term symbol usually follows the electron configurationIf n=3 and l=0, it is the 3s subshell, and so Thus the s subshell has only one orbital, the p subshell has three orbitals, and so on 4 Spin Quantum Number (m s) m s = ½ or ½ Specifies the orientation of the spin axis of an electron An electron can spin inIn the figure below, we see examples of two orbitals the p orbital (blue) and the s orbital (red) The red s orbital is a 1s orbital To picture a 2s orbital, imagine a layer similar to a cross section of a jawbreaker around the circle The layers are depicting the atoms angular nodes To picture a 3s orbital, imagine another layer around the


Spdf Orbitals Can Hold How Many Electrons Qecs Bamagien Site


Sublevel Spdf Chart Fgkb Katasekan Site
Although there is no pattern in the first four letters (s, p, d, f), the letters progress alphabetically from that point (g, h, and so on)Some of the allowed combinations of the n and l quantum numbers are shown in the figure below The third rule limiting allowed combinations of the n, l, and m quantum numbers has an important consequence It forces the number of subshells in a shell to beThe simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively These names, together with the value of n , are used to describe the electron configurations of atomsThe empty f orbitals in lanthanum, actinium, and thorium contribute to chemical bonding, as do the empty p orbitals in transition metals 32 Vacant s, d, and f orbitals have been shown explicitly, as is occasionally done, 33 to emphasise the filling order and to clarify that even orbitals unoccupied in the ground state (eg lanthanum 4f or



Quantum Number Wikipedia



2 4 Electron Configurations Chemistry Libretexts
The first two combinations were assigned the partial term of S 6 As e α 2 and e β were given an P symbol, the combination of them gives their direct product P × P = S P D The direct product for e α and e β 2 is also P × P = S P D Considering the degeneracy, eventually the term symbols for p 3 configuration are 4 S, 2 DSublevels are commonly given letter designations The l = 0,1,2,3,4,5, sublevels are designated as s, p, d, f, g, sublevels, respectively For known elements no value of l higher than 3 (f sublevel) is necessary Two quantum numbers (n and l) are required to specify a particular energy sublevel The magnetic quantum number, m l


Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
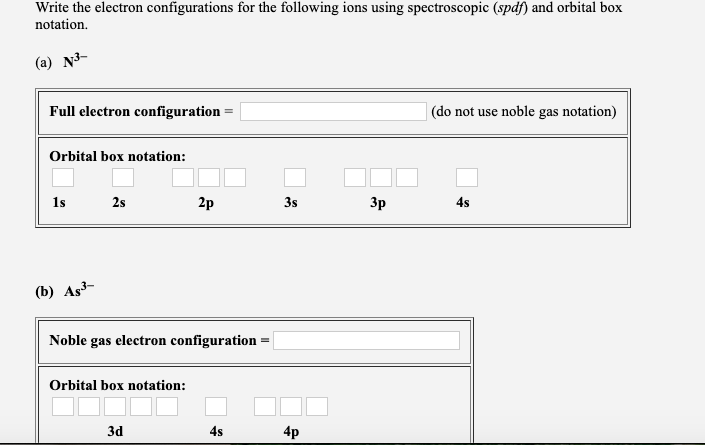


Solved Write The Electron Configurations For The Followin Chegg Com


An Atomic Model Our Present Model Of The Atom Is Based On The Concept Of Energy Levels For Electrons Within An Atom And On The Mathematical Interpretation Of Detailed Atomic Spectra The Requirements For Our Model Are Each Electron In A Particular Atom
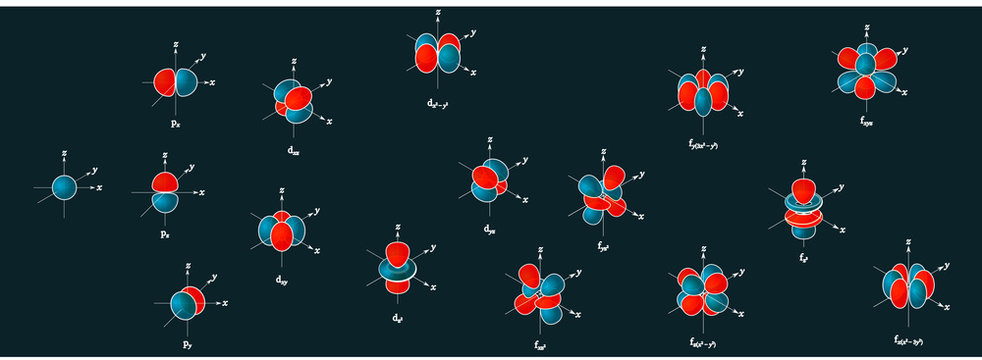


D Orbital Stock Photos And Royalty Free Images Vectors And Illustrations Adobe Stock



Parsing The Spdf Electron Orbital Model Chemistry Education Chemistry Classroom Teaching Chemistry



S P D F Block Chemistry Midterm 1 Flashcards Quizlet



Orbitals Diagram For Spdf Quantum Numbers In High School Chemistry Physics And Mathematics Chemistry Classroom Teaching Chemistry
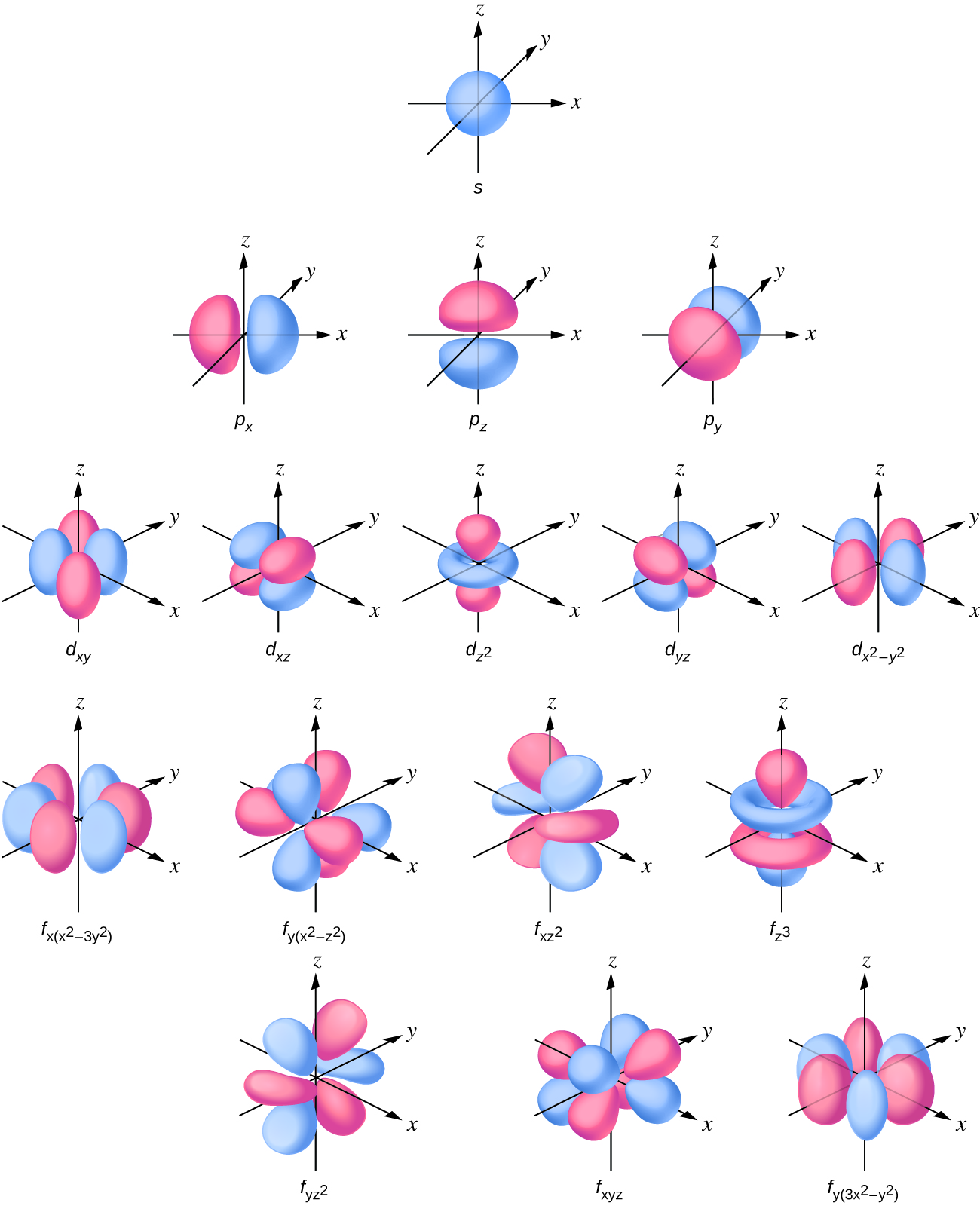


2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts
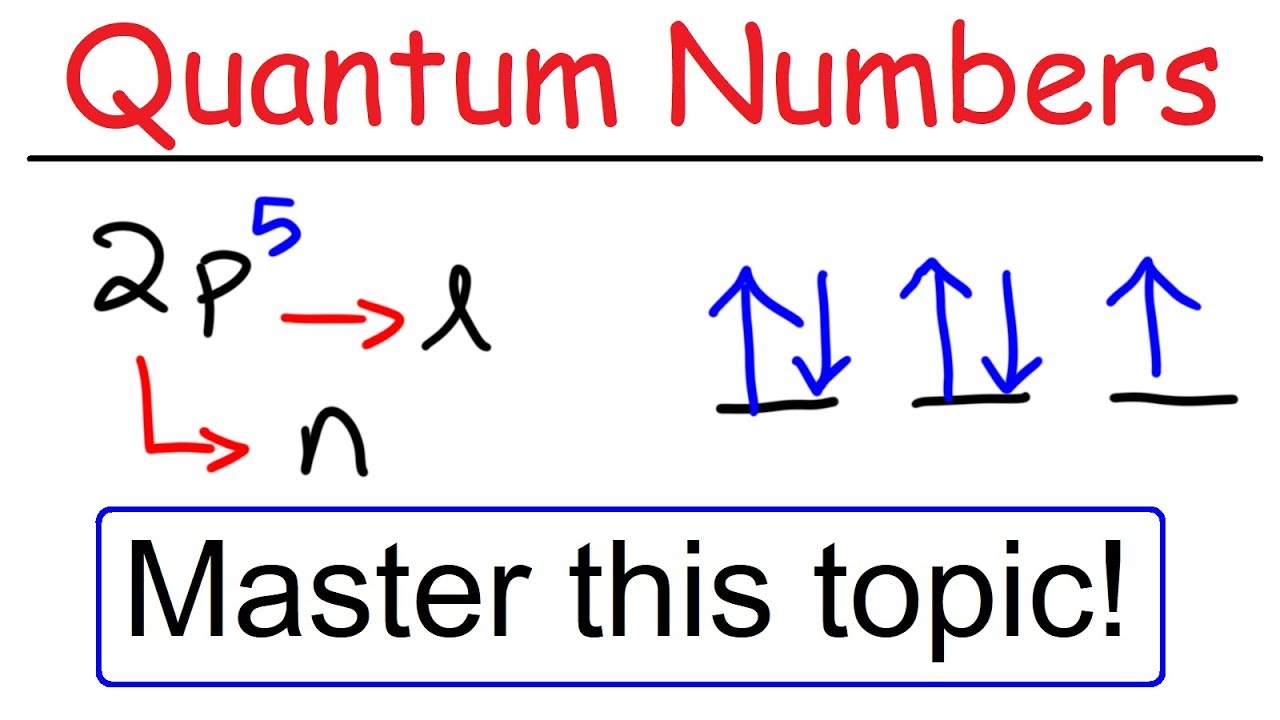


Quantum Numbers N L Ml Ms Spdf Orbitals Youtube



Basic Bohr To Quantum Orbitals Klm To Spdf Ppt Download


Is There Any Difference In Energy In Orbitals S P D And F Quora
:max_bytes(150000):strip_icc()/antibonding-5b54ef9046e0fb005b6d11a9.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers



Chemistry Models Of The Atom Flashcards Quizlet


Gsjournal Net Science Journals Research papers Chemistry Download 5032


Vixra Org Pdf 1308 0130v1 Pdf


Parsing Spdf Orbital Hybridization And Simple Bonding



Pin By Fernando Visintin On Science Physical Chemistry Electron Configuration Teaching Chemistry


Parsing The Spdf Electron Orbital Model



S P D F Orbitals Chemistry Socratic
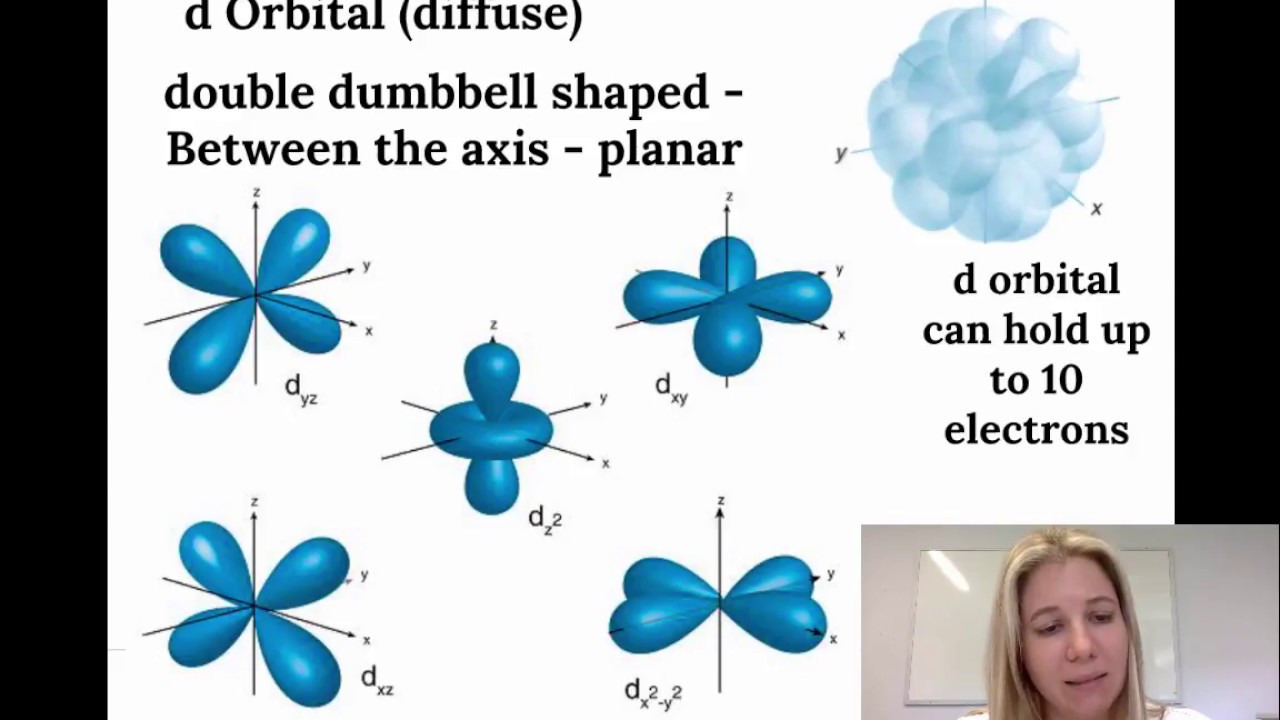


Electron Configuration Spdf Notation Part 1 Youtube



Powerpoint Orbital Shape Orientation Spdf Periodic Table Powerpoint Presentation Free Online Download Ppt 6tz333


Parsing The Spdf Electron Orbital Model
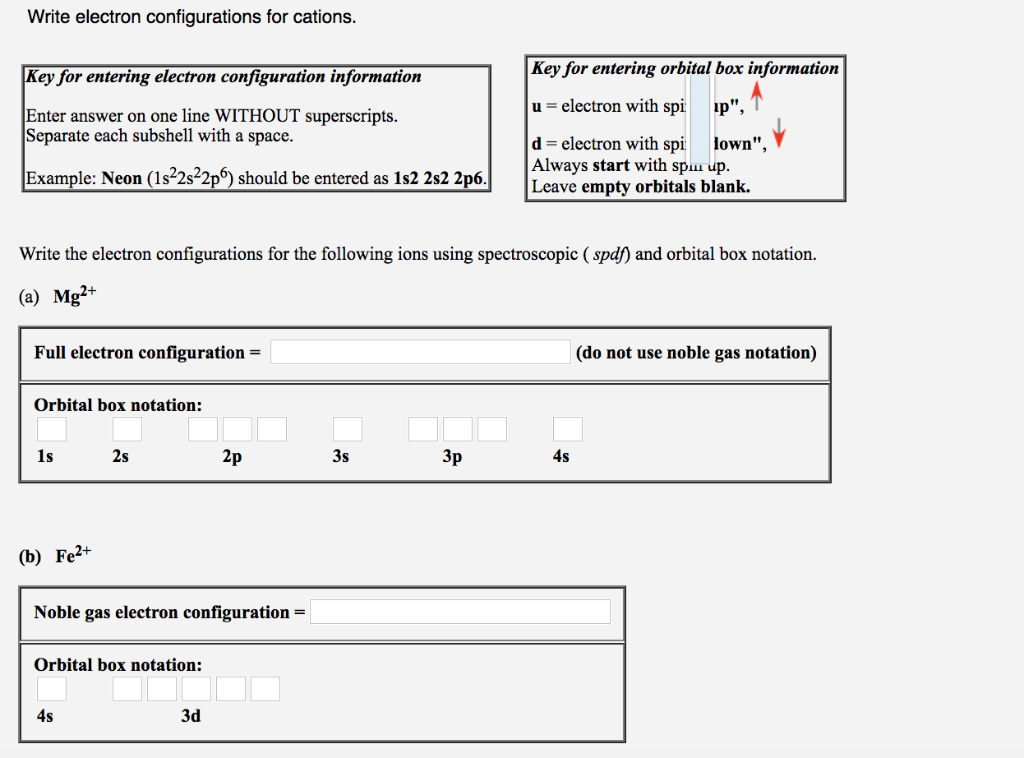


Solved Write The Electron Configurations For The Followin Chegg Com
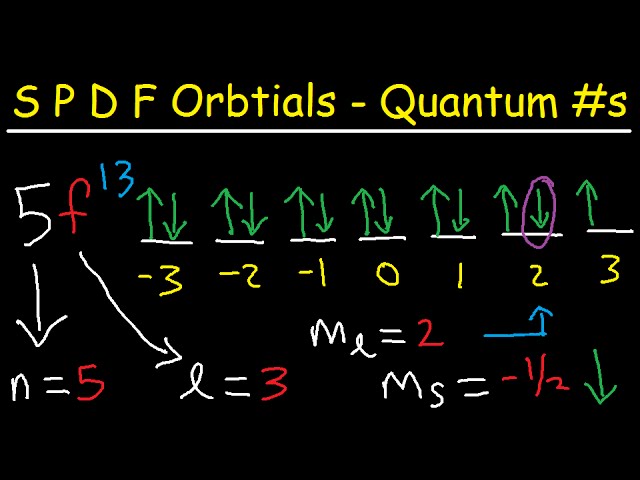


S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube


Electron Configurations



What Is Spdf Configuration Chemistry Stack Exchange


3


Parsing Spdf Orbital Hybridization And Simple Bonding
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


Electron Configuration Chart



Electron Configuration Wikipedia


Q Tbn And9gcth1rc3hbnde1titk095wzz5fdzyo5obndscg8azgis25 Lq4re Usqp Cau



Electron Configuration Spdf Notation Part 2 Youtube


Parsing The Spdf Electron Orbital Model


Parsing Spdf Orbital Hybridization And Simple Bonding



Ninth Grade Lesson Introduction To Electron Orbital Levels
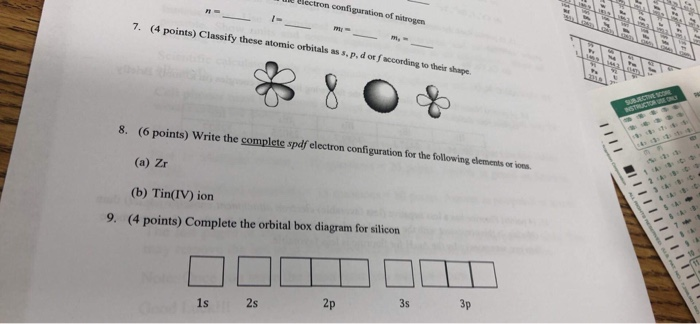


Solved These Atomic Orbitals As S P D Ot Faccording To Chegg Com
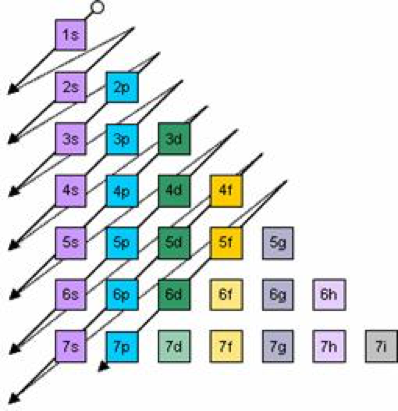


Electron Configurations Orbitals Energy Levels And Ionisation Energy Trends A Level Chemistry Revision Notes
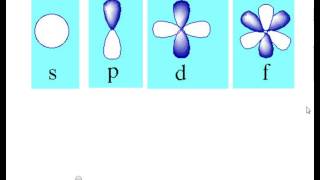


Quantum Model And Spdf Orbitals Youtube
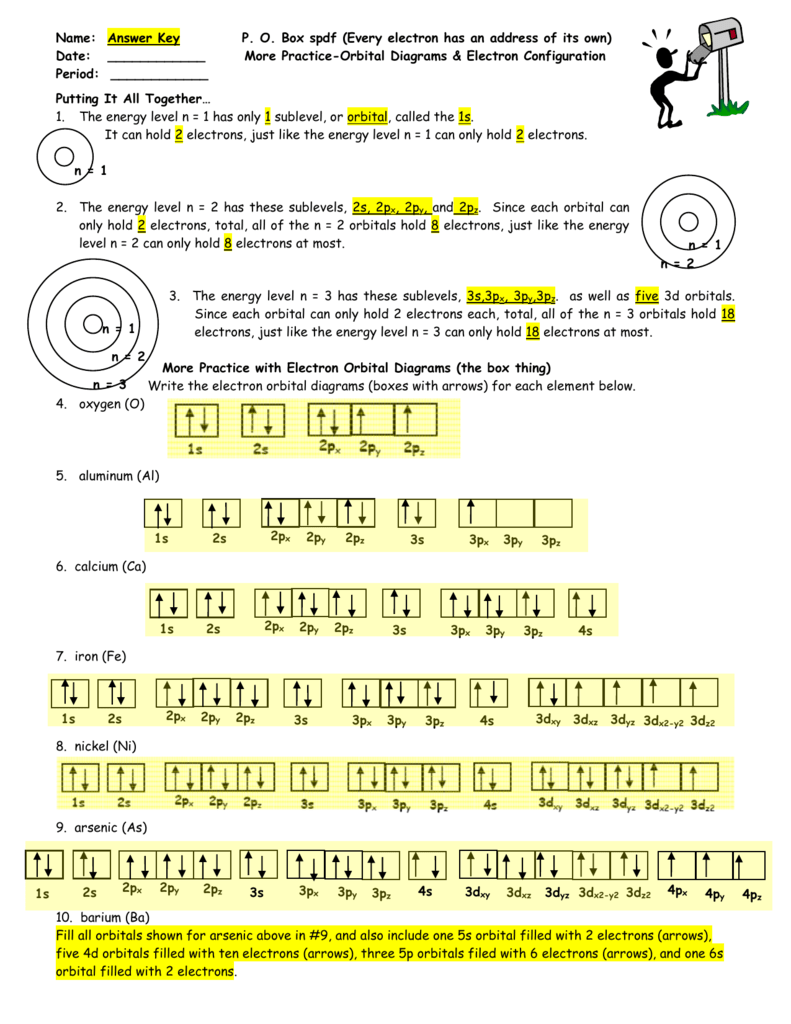


Po Box Spdf Worksheet Answer Key


Orbitals And Electron Configuration



Quantum Numbers The Easy Way Youtube



File Atomic Orbitals Spdf M Eigenstates And Superpositions Png Wikimedia Commons
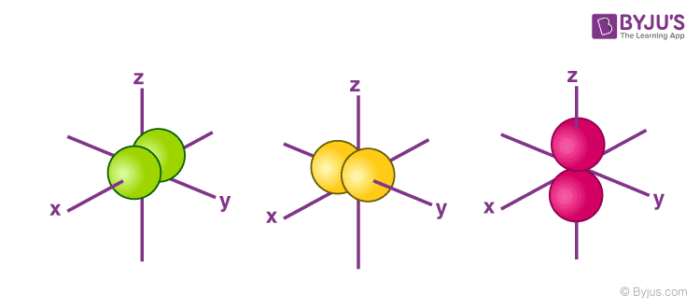


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital



What Do S P D And F Mean In Chemistry And Why Do You Need Them Chemistry Quantum Momentum
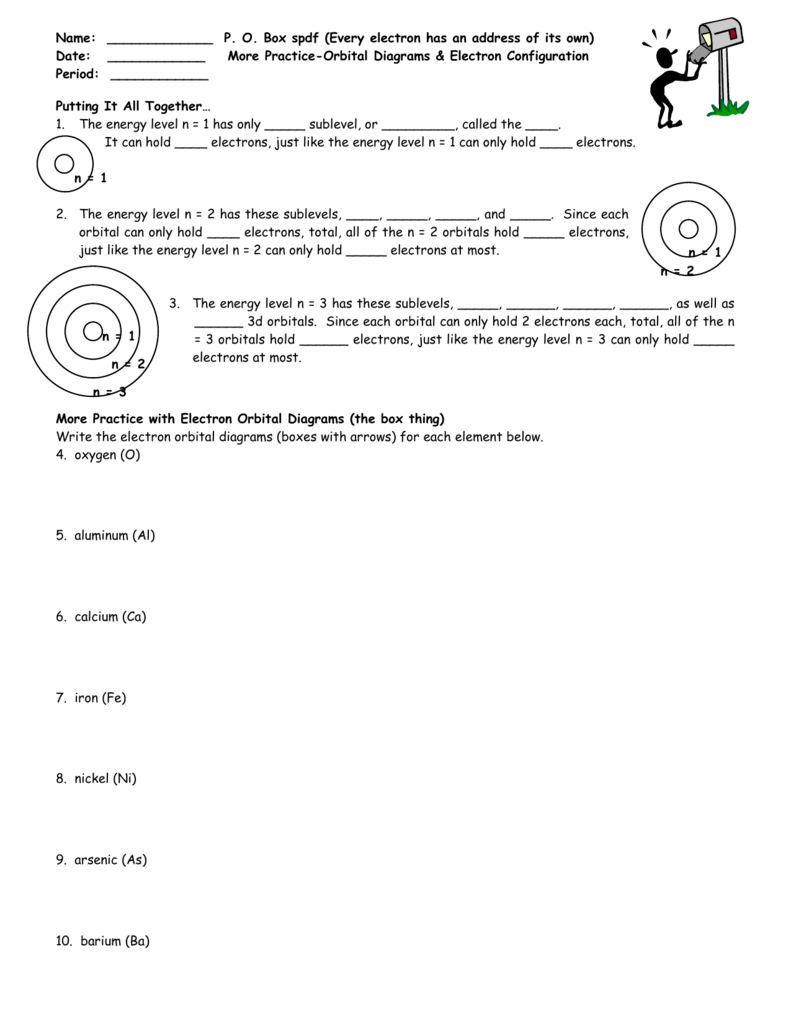


Po Box Spdf Worksheet


Q Tbn And9gcqkmfnyiafrl15xmtugyzntdsh3bes4t5 Mafe1zuzdwzpff2xm Usqp Cau



Origin Of Spdf Orbitals Youtube


Difference Between S Orbital And P Orbital Definition Shape Structural Properties


Basic Bohr To Quantum Orbitals Klm To Spdf Ppt Download


Visualizing Electron Orbitals


Electron Configurations


Parsing The Spdf Electron Orbital Model



Write The Electron Configurations For P And Cl Using Both Sp Clutch Prep


How Do You Draw S P D F Orbitals Socratic


Parsing Spdf Orbital Hybridization And Simple Bonding
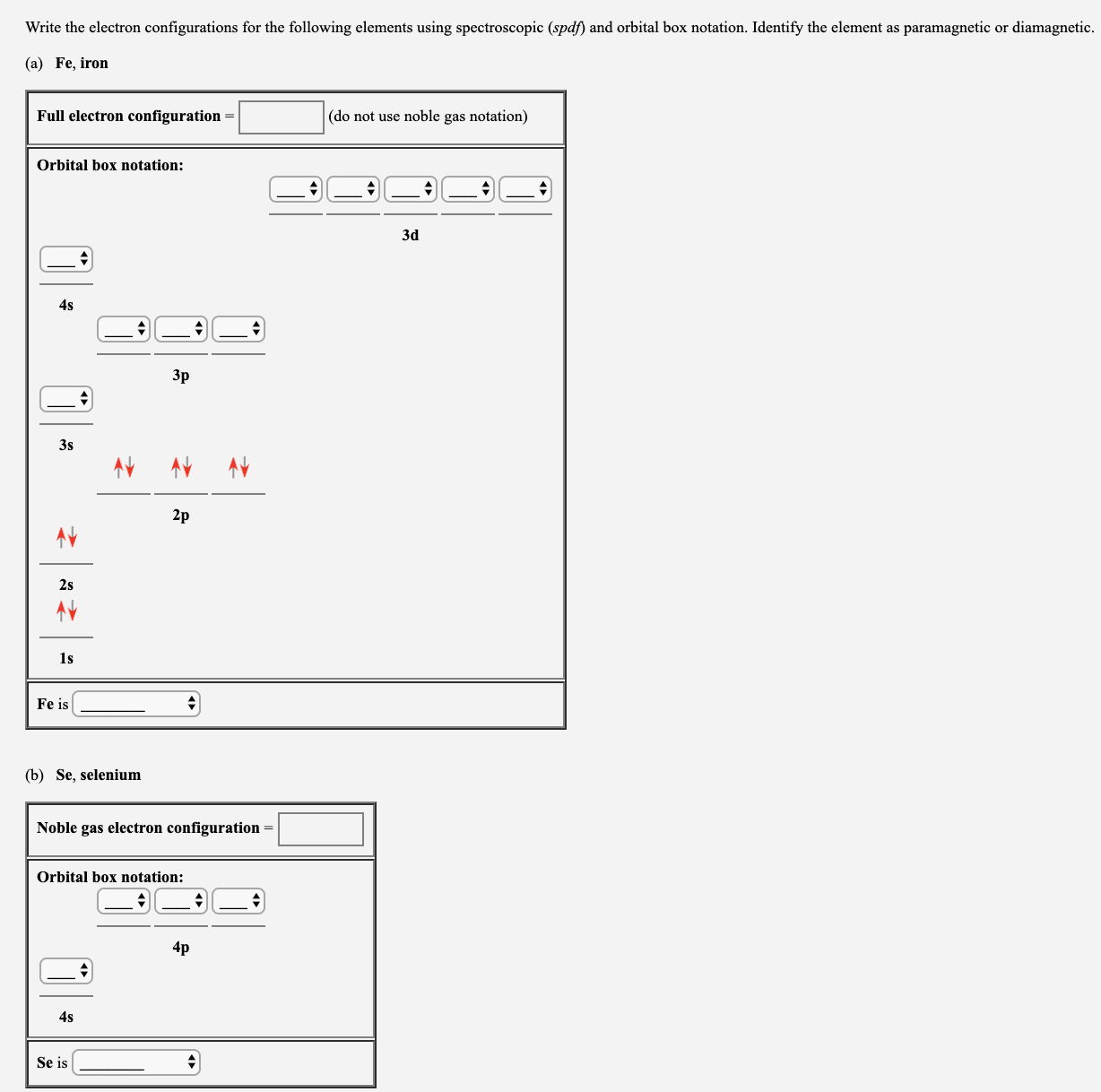


Answered Write The Electron Configurations For Bartleby



Oneclass Write The Electron Configurations For Mg And Ar Using Both Spdf Notation And Orbital Box Di


S P D F Orbitals Chemistry Socratic



Orbitals The Basics Atomic Orbital Tutorial Probability Shapes Energy Crash Chemistry Academy Youtube
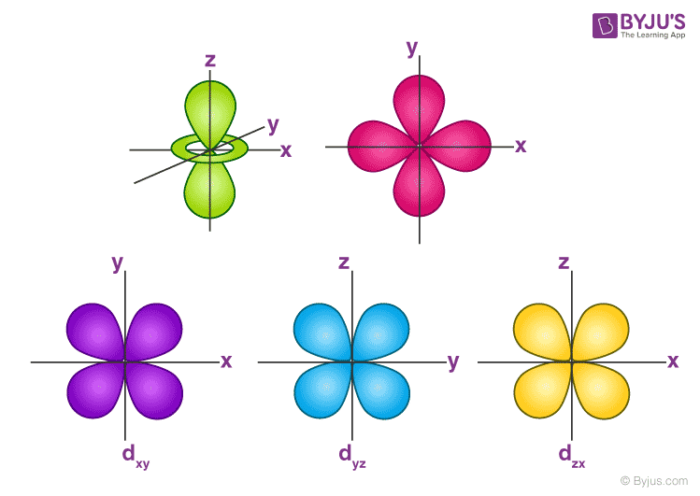


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital
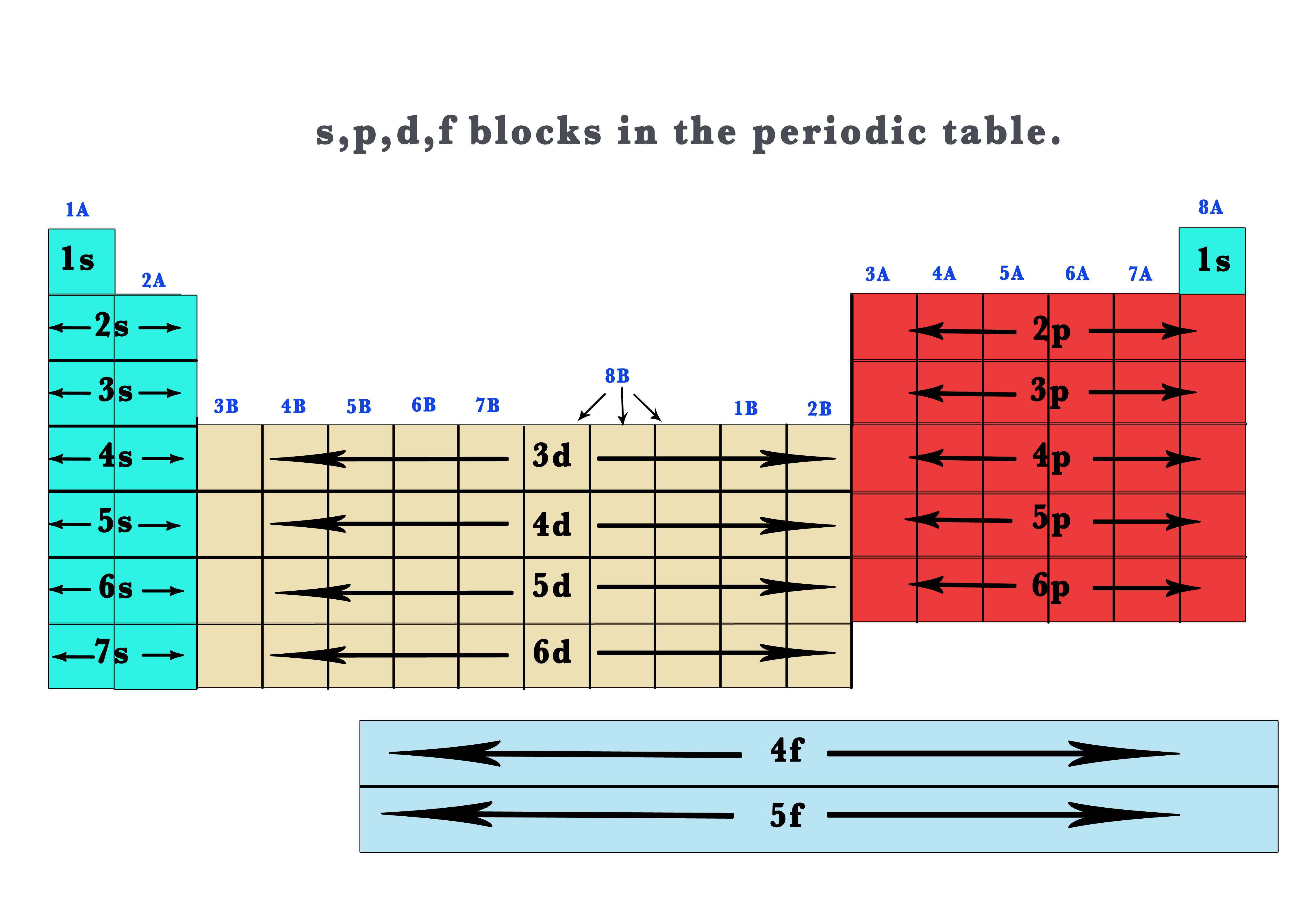


41 The Periodic Table S P D F Blocks Madoverchemistry Com
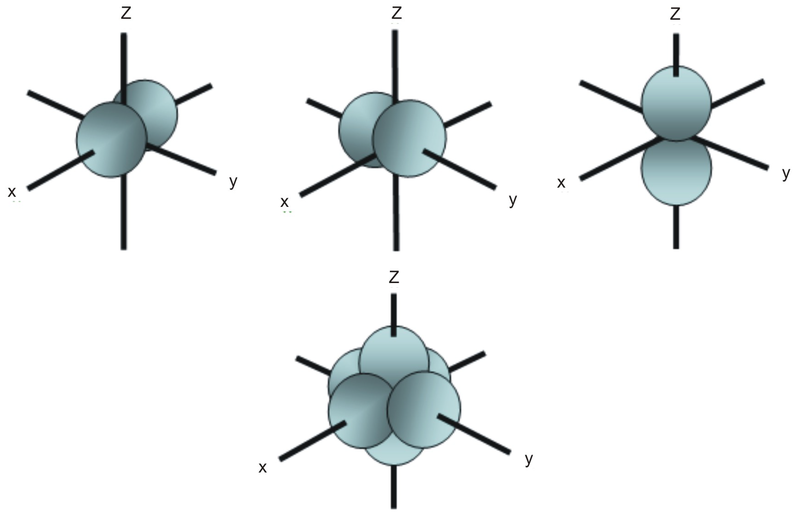


S P D F Orbitals Chemistry Socratic


Shapes Of Orbitals And Sublevels



A Chart Of The Spdf Electron Orbitals Chemistry Education Chemistry Classroom Teaching Chemistry
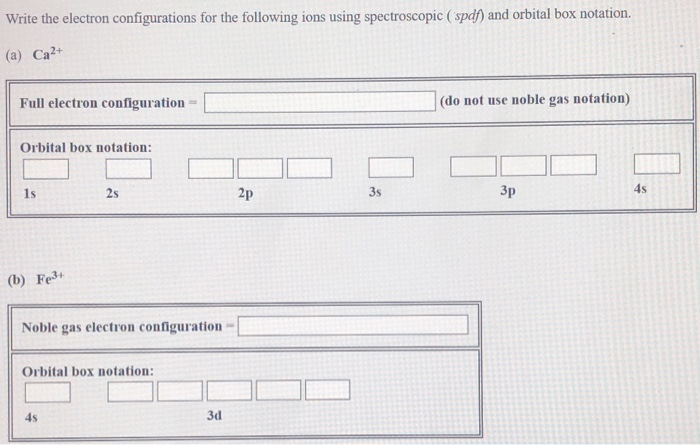


Solved Write The Electron Configurations For The Followin Chegg Com


Shape Of Orbitals Spdf Download Online Games


Ppt S P D F Orbitals Powerpoint Presentation Free Download Id


How Many Electrons Can S P D F Orbitals Hold S Bravo Art Back Pain Doctor Delray Beach Utilities


Parsing Spdf Orbital Hybridization And Simple Bonding



The Atomic Spectrum Of Hydrogen Orbitals And Spdf Notation Electromagnetic Spectrum Emission Spectrum


コメント
コメントを投稿